Nature | News Feature
To dissect evolution, Joe Thornton resurrects proteins that have been extinct for many millions of years. His findings rebut creationists and challenge polluters.

SHAWN RECORDS
One deep-frozen vial holds the more-than-600-million-year-old ancestor of the receptors for oestrogen, cortisol and other hormones, which Thornton brought to life1 nine years ago. Other tubes house proteins more than 400 million years old, which Thornton resurrected a few years later to show how an ancient receptor had changed its preferences — and how the march of evolution cannot be reversed2, 3, 4. In another corner of the freezer rest the ancient protein components of a sophisticated cellular machine that acquired a more complex form through random mutations rather than selection for superior function, as the group showed in Nature this January5. The sheer awe of working with long-dead proteins doesn't fade, says Thornton. “It's amazing. The ability to do this type of time travel is fantastic.”
Thornton is a leader in a movement to do for proteins what the scientists in Jurassic Park did for dinosaurs: bring ancient forms back to life, so that they can be studied in the flesh. “Instead of passively observing things as most evolutionary biologists do, you actively go in and test the hypotheses experimentally,” says Antony Dean, a molecular biologist at the University of Minnesota in St Paul who heads another major group in the field. “His is one of the leading labs, no doubt.” And Thornton is tackling some important questions, says Kenneth Miller, a molecular biologist at Brown University in Providence, Rhode Island. “He's helping to put some flesh on the bones of speculation about how complexity arises.”
What isn't so widely known is that evolutionary biology is Thornton's second career: in his first, he was an activist for Greenpeace, campaigning vigorously against the release of toxic chemicals. He wrote a controversial book on organochlorines: industrial chemicals that include dioxins, polychlorinated biphenyls (PCBs) and pesticides such as DDT. That activist legacy bleeds into his work today, for example in his focus on the oestrogen receptor, which is corrupted by many pollutants. The grubby, sea-green tiles under Thornton's lab benches were carefully sourced to be free of polyvinyl chloride (PVC), one of the organochlorines that worries him most. His activist past also helps to explain why he has been fearless — almost enthusiastic — about highlighting the challenge that his work presents to a creationist argument called intelligent design: the claim that complex molecular systems can only have been created by a divine force. Thornton shows how evolution did the job, leaving no need for a designer.
Environment to evolution
Thornton says that his activist days — during which he saw that many risk-assessment models were shot through with assumptions and biases — left him “intensely committed to methodological reductionism and experimentalism”, which he now uses to break evolution down into detailed steps that he can test. “If you're doing science, I think it ought to be as strong and decisive as possible,” he says. “If you're doing politics, go ahead, but don't try to disguise it as science.”Thornton's unconventional career path started with an obsession with Moby Dick, which led him to study English at Yale University in New Haven, Connecticut. But the course, with its focus on the philosophy of criticism rather than literary texts, left him with a hunger for reality, and nothing seemed more real than politics and activism. He dropped out of college, signed up with Greenpeace and spent several months doorstepping to canvass people for money and support.
Nature Podcast
Helen Pearson talks about Joe Thornton and his protein resurrection labBut Thornton was growing older, and yearning to “develop my own body of work”. His time with Greenpeace had taught him the power of science to influence society, and his ambitions turned to research. First, he had to deal with the small matter of graduating from Yale. Then living in New York, he did that by accruing course credits at Columbia University — attending his first molecular-biology classes aged 30 — only to find himself rejected from almost every graduate programme he applied to, in part because of his unusual CV.
Of the seven friends and colleagues of Thornton's who spoke to Nature, six called him intense. The seventh described him as “beyond intense”. But only a little of that intensity is apparent at his Wednesday morning lab meeting in Eugene. The freezer crisis has blown over: the power came back after half an hour and the thermometer rose to only −76 °C. Now graduate student Dave Anderson gets a friendly grilling during a practice talk outlining his thesis proposal: to trace the evolution of the DNA-binding domain of an ancient hormone receptor. The meeting stretches on for 2.5 hours — not uncommon in this lab, everyone says.
A binding fascination
Since his Greenpeace days, Thornton has been fascinated by the steroid hormone receptors: in vertebrates, six proteins that sit in the cell nucleus and control the activity of genes. By binding specific 'ligands' — hormones ranging from oestrogens and androgens to cortisol — the receptors trigger “these remarkable cascades of biological activity during development and physiology”, Thornton says. “Their affinity for their hormones is just stunning. A drop of hormone in a railroad tank car of serum is enough” — and yet, as Thornton learned at Greenpeace, they can be waylaid by toxic substances. “I wanted to know where that system came from,” he says.When he was finally accepted for a PhD at Columbia, he set about comparing receptor genes from living organisms to piece together a detailed history of how the receptor family had evolved6.
Nature Podcast
Joe Thornton describes how a molecular machine evolved greater complexityThe book caused a stir. Drawing on arguments that he had formulated at Greenpeace, Thornton made the case that regulatory policy should focus on managing classes of toxic chemicals rather than tens of thousands of substances, one by one — and that the priority should be organochlorines. These substances, generated by the use of chlorine gas in the chemical and paper-making industries, have properties of stability and solubility that make them desirable to industry but problematic to the environment because they are long-lived and accumulate in animal tissues. Nature's review called Pandora's Poison a “landmark” and another review compared it to Rachel Carson's famous 1962 treatise on pollutants, Silent Spring. The Chlorine Chemistry Council in Washington DC, however, decried Thornton's “hyperbole and faulty risk analysis”.
But Thornton was already gearing up to make a different kind of splash, with his first paper in Science1. He and his team trampled the assumption that only vertebrates have steroid hormone receptors by cloning one from the sea slug Aplysia californica. The finding implied that the origin of the receptor gene was far more ancient than anyone had realized. “I would've hated to be a fellow grad student. He was writing a book and publishing in Science and having two children at the same time,” says Darcy Kelley, a biologist at Columbia and Thornton's other PhD co-supervisor.
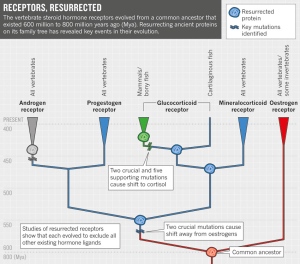
The approach that Thornton took in the 2003 study is one that he has loosely followed ever since. Starting with the genes for steroid hormone receptors from a slew of living organisms, he clambered backwards through the evolutionary tree to deduce the most likely sequence of the common ancestor of all such receptors, which existed some 600 million to 800 million years ago, in the common ancestor of “you and a snail”, as he puts it. Instead of stopping there, as most evolutionary biologists would have done, he then built the gene and inserted it into cells that could manufacture the ancient protein.
Resurrecting the protein, says Thornton, allowed his team “to experimentally test hypotheses about evolution that would otherwise be just speculation”. They went on to show1 that the ancestral receptor was sensitive to oestrogens but not to related hormones — supporting the idea that the family of receptors evolved through a series of gene duplications and that the copies gradually evolved affinities for other ligands (see 'Receptors, resurrected').
By the time his paper came out in Science, Thornton had taken a faculty position in Eugene, an old hippy town that pays as much homage to bicycles as it does to cars. He built a house (no PVC, sustainable bamboo floors) and set to work building up his protein-resurrection lab.
Thornton wanted to delve deeper into the puzzle of how complex systems with tightly interacting molecular parts evolve. It was a long-standing conundrum. As Charles Darwin wrote in On the Origin of Species: “If it could be demonstrated that any complex organ existed which could not possibly have been formed by numerous, successive, slight modifications, my theory would absolutely break down.” And what was an evolutionary puzzle to biologists was a target for evolution's critics. Michael Behe, a biochemist at Lehigh University in Bethlehem, Pennsylvania, and a senior fellow at the Discovery Institute in Seattle, Washington, proposed in the 1990s that such systems — the blood-clotting cascade, for example, or the molecular motor called the flagellum — are so “irreducibly complex” that they could not have evolved step by step, and can only be the product of intelligent design.
Thornton says that he didn't set out to refute intelligent design, but the prospect of a fight hardly put him off. “Been there, enjoyed that,” he says. He chose to explore a pair of steroid hormone receptors: the mineralocorticoid receptor (MR), which binds the hormone aldosterone and regulates salt and water balance; and the closely related glucocorticoid receptor (GR), which binds cortisol and controls stress response. A gene duplication more than 450 million years ago produced the two receptors — but aldosterone didn't arise until many millions of years later. The timing seemed to make the MR a textbook example of irreducible complexity: how could selection drive the evolution of a lock (the MR) to fit a key (aldosterone) that didn't yet exist?
Evolution at work
Led by Bridgham, Thornton's team found the answer by resurrecting the ancestor of both receptors. To their surprise, it was sensitive to aldosterone, suggesting that it had been activated by an ancient ligand with a similar structure2. Once aldosterone had evolved, the team proposed, evolution was able to take advantage of the existing receptor to control a new biological function — a process that Thornton termed molecular exploitation. They also showed how its sister receptor, the GR, was evolving functions of its own.“Such studies solidly refute all parts of the intelligent design argument,” wrote Christoph Adami, an evolutionary biologist at the Keck Graduate Institute of Applied Life Sciences in Claremont, California, in an article entitled 'Reducible complexity'7. But Behe dismissed the result. The receptor and ligand are not irreducibly complex, he says, and evolution did not give them any truly new function. “I think his results are quite consistent with my own view that Darwinian processes are poor ones to explain the complexity found in life,” Behe told Nature.
“He's helping to put some flesh on the bones of speculation about how complexity arises.”
In a final chapter to the story, Thornton tried to run that evolutionary sequence backwards. But when the researchers reversed the seven mutations in the ancient cortisol-specific form, they could not transform it back into a protein that worked like the common ancestor of the GR and MR. They instead engineered a dud, unable to respond to any hormone4. That was because a handful of other mutations had also cropped up on the way to making a cortisol-specific receptor. They played little part in the receptor's new function, but acted as an evolutionary ratchet, preventing it from regaining its old one.
Thornton showed that it was necessary to undo those mutations too, to reverse the change. To him, the work was a powerful demonstration that the path of evolution can be contingent on random events. “Chance plays a very large role in determining what evolutionary outcomes are possible,” he says. The study captivated the scientific press — and beyond. “Evolution opens gateways into the future. But it appears to close them — firmly — behind it as well,” read an editorial in the New York Times.
In the Nature article that was published this year5, Thornton took a break from hormone receptors, and instead collaborated with Tom Stevens, a geneticist at Eugene, to dissect the evolution of V-ATPase, a molecular machine that pumps protons across membranes to acidify compartments inside cells. The group wanted to know how an essential part of the machine — a ring of proteins that spans cell membranes — evolved from an ancestral form with two components to one with three.
With their protein-resurrection toolbox, the researchers showed that, around 800 million years ago, the ancestral gene coding for one protein component was duplicated, and the daughter genes then picked up two vital mutations. The changes meant that the proteins could no longer sit anywhere in the ring, but instead had to occupy a specific spot. Suddenly, the ring could function only with all three parts. What surprised Thornton was that the three-component ring seemed to work no better than its two-component counterpart. Random mutations that actually corrupted proteins had led to 'irreducible complexity'.
Computing complexity
The study flipped another finger to intelligent-design proponents — but “I'm sort of bored with them”, Thornton says. He is more excited by the next scientific story that is about to come out of the lab. His group wanted to explore how the ancestor of the entire steroid hormone receptor family, which was sensitive only to oestrogens, evolved into forms sensitive to other hormones. And this time, he found no clues in the crystal structures of resurrected proteins from before and after the change.The answer can be found on a computer screen at the end of Thornton's lab. Mike Harms, a postdoc who joined the lab three years ago, used his expertise in biophysics and some immense computational power to simulate the movements of every atom in the ancestral receptors, showing how just two mutations drove the transformation. When Harms hits play, an oestrogen molecule snuggles its way into the binding pocket of a receptor roughly 550 million years old. But when he runs a simulation of the same receptor with those two mutations, the oestrogen never finds a comfortable spot.
This evolutionary story also sheds light on why the oestrogen receptor is now vulnerable to the threats against which Thornton campaigned in his former life. The team worked out that each steroid receptor evolved to be only as specific as it had to be to bind its target ligand and exclude all others that existed at the time. The oestrogen receptor achieves this by binding substances that contain a chemical structure called an aromatized A ring. Because oestrogens are the only steroid hormones to have such a ring, that criterion was enough to ensure that the receptor bound only oestrogens for many millions of years. Until, that is, the chemical industry started pumping out hundreds of substances containing such aromatized rings, which the oestrogen receptor unwittingly bound. “The endocrine disrupters are taking advantage, unfortunately, of the promiscuity that is the result of the evolutionary history of receptors,” Thornton says.
Thornton does see progress on the issues on which he once campaigned. Production of toxic chemicals in the United States has fallen since his days with Greenpeace, and in 2007 the European Union enacted REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals), which emphasizes elimination of the most dangerous substances. That law puts the onus on the chemical industry to show that a chemical is safe rather than on regulators to prove it is dangerous — the approach for which Thornton argued in Pandora's Poison.
Does he miss having something to campaign against? Yes and no. “I'm less able to convince myself that the world has to be exactly as I envision it. So it's harder for me to occupy that activist persona.” Besides, he says, “My kids take all that energy now.”
Or almost. His creations need tending too. Back in his office, we listen to the tinny voicemail message left by the freezer on his phone earlier that day. “The past is calling,” Thornton says.
- Nature
- 483,
- 390–393
- ()
- doi:10.1038/483390a
No hay comentarios:
Publicar un comentario